China Scholarship Scheme with The University of Manchester
4 year fully funded PhD project with Professor Patrick O’Malley, School of Chemistry
Understanding Photosynthetic Solar Energy Conversion: Modelling The Charge Separated State and the Oxygen Evolving Complex in Photosynthesis
By the year 2050 even the most conservative of estimates predict the world’s energy requirements will double. Energy is a prerequisite to economic stability and its supply at an affordable cost for both the developed and developing world needs to be guaranteed. Increased use of fossil fuels brings about unacceptable environmental effects via CO2 emissions with consequent dire results for runaway global warming. There is now acceptance that we need a radical reassessment of how we generate future energy requirement. As enough solar energy reaches the earth in an hour to supply the world’s annual energy consumption needs. The technological problem is how to convert this incident solar energy into forms usable by mankind (1).
Nature, via photosynthesis, has for around 3 billion years come up with elegant solutions to achieve this goal and by learning to imitate it using artificial systems it is now believed that we can guarantee the energy requirements of future generations (2).
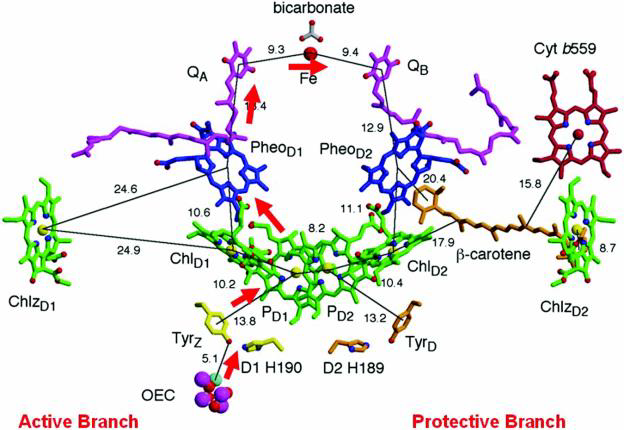
Solar energy is converted and stored as chemical energy in photosynthetic organisms by capturing the sun’s visible light energy and using it to generate a charge separation potential across the chloroplast or bacterial membrane This generated potential is later used to provide the energy source for the conversion of carbon dioxide to carbohydrate which can be used by the organism to supply its energy requirements during respiration. The efficient storage of solar energy therefore requires that electronic excitation energy be efficiently converted into chemical energy by a fast electron transfer event leading to a long-lived charge separation. In photosynthesis the initial charge separated state is formed via rapid electron transfer steps from a singlet excited state chlorophyll molecule on one side of the membrane to a quinone molecule situated on the opposite side of the membrane. The charge separated state so-formed consists of a chlorophyll cation free radical and a semiquinone anion free radical. The surrounding protein environment plays a crucial role in catalysing the efficient electron transfer across the membrane and in stabilising the chlorophyll and semiquinone species to maintain the charge separated state.
This project aims to use Density Funtional Theory (DFT) molecular modeling methods to provide an understanding of how the protein environment of these cofactors facilitates electron transfer and also helps stabilise these radical species to maintain a charge separated state. The techniques used will include electronic structure calculation based on broken symmetry DFT calculations (3,4). Parallel experimental studies using Electron Paramagnetic Resonance (EPR) will be performed allowing calculated EPR parameters to be compared with experimental observables. In such a fashion a solid link to experimentally observed data will set a limit on proposed theoretical intermediates. Using crystallographically determined coordinates for the cofactor and protein atoms the stability and spectroscopic characteristics of the charge separated state will be revealed. The studies will focus in particular on the manganese calcium oxo complex which catalyses the important conversion of water to hydrogen and molecular oxygen. With such fundamental an understanding we can then proceed to intelligently design artificial systems to mimic nature’s method of solar energy capture.
1. Photoconversion of Solar Energy, Molecular to Global photosynthesis, Imperial College Press, London, 2004.
2. Harnessing Solar Energy for the Production of Fuels, European Science Foundation Report, 2006.
3. “Hydrogen Bond Network around the Semiquinone of the Secondary Quinone Acceptor QB in Bacterial Photosynthetic Reaction Centers” Taguchi, Alexander T.; O'Malley*, Patrick J.; Wraight, Colin A. Journal of Physical Chemistry B, (2015), 119, 5805-5814.
4. “Manganese Oxidation State Assignment for the Manganese Catalase”, Beal, Nathan. J., O’Malley* Patrick J., J Amer Chem Soc, (2016), 138, 4358 – 4361.
附件为:UoM & CSC Scholarship Award Application Form 2017/18 entry
Professor Patrick O’Malley邮箱: patrick.o'malley@manchester.ac.uk